Titration Curve How To Read . how do you explain the shape of a titration curve? You will also learn about equivalence points and. And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Everything you need to know for a level. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. Strong acid/strong base titration curves. The equivalence point of a. The shape of the graph produced is called a titration. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution.
from www.numerade.com
You will also learn about equivalence points and. a titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a level. a titration curve is a graphical representation of the ph of a solution during a titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. And why is the equivalence point not always at ph7? The equivalence point of a. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. The figure below shows two different examples of a. Strong acid/strong base titration curves.
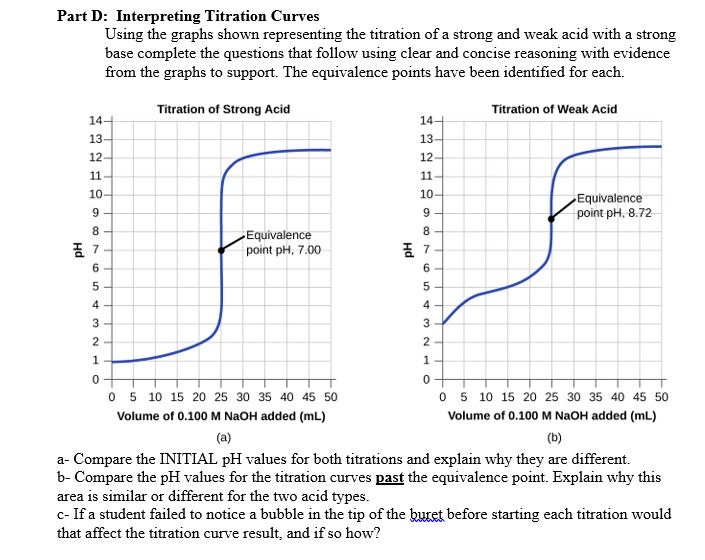
SOLVED Part D Interpreting Titration Curves Using the graphs shown
Titration Curve How To Read And why is the equivalence point not always at ph7? Everything you need to know for a level. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a. Strong acid/strong base titration curves. a titration curve is a graphical representation of the ph of a solution during a titration. The shape of the graph produced is called a titration. how do you explain the shape of a titration curve? a titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a. You will also learn about equivalence points and. And why is the equivalence point not always at ph7?
From narodnatribuna.info
Ppt How To Interpret Titration Curves Powerpoint Titration Curve How To Read in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The equivalence point of a. how do you explain the shape of a titration. Titration Curve How To Read.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Curve How To Read how do you explain the shape of a titration curve? Strong acid/strong base titration curves. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. a titration curve is a graphical representation of the ph of a solution during a titration. The. Titration Curve How To Read.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Titration Curve How To Read The equivalence point of a. Everything you need to know for a level. how do you explain the shape of a titration curve? Strong acid/strong base titration curves. The figure below shows two different examples of a. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way. Titration Curve How To Read.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve How To Read how do you explain the shape of a titration curve? You will also learn about equivalence points and. Strong acid/strong base titration curves. a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? Everything you need to know for a level. . Titration Curve How To Read.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration Curve How To Read The shape of the graph produced is called a titration. Everything you need to know for a level. The equivalence point of a. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. how do. Titration Curve How To Read.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Curve How To Read a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. a titration curve is a graphical representation of. Titration Curve How To Read.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve How To Read Everything you need to know for a level. The figure below shows two different examples of a. a titration curve is a graphical representation of the ph of a solution during a titration. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. Strong acid/strong. Titration Curve How To Read.
From www.youtube.com
How to draw acid base Titration Curve and selection of Suitable Titration Curve How To Read how do you explain the shape of a titration curve? The figure below shows two different examples of a. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. You will also learn about equivalence points and. a titration curve is a graphical representation. Titration Curve How To Read.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve How To Read The equivalence point of a. how do you explain the shape of a titration curve? The figure below shows two different examples of a. a titration curve is a graphical representation of the ph of a solution during a titration. You will also learn about equivalence points and. during a titration, ph can be plotted against the. Titration Curve How To Read.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve How To Read a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? how do you explain the shape of a titration curve? Everything you need to know for a level. The shape of the graph produced is called a titration. during a titration,. Titration Curve How To Read.
From studylib.net
How to Interpret Titration Curves How to Interpret Titration Curves Titration Curve How To Read a titration curve is a graphical representation of the ph of a solution during a titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Everything you need to know for a level. The figure below shows two different examples of a. Strong acid/strong base titration curves. The shape. Titration Curve How To Read.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve How To Read during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a. The equivalence point of a. Everything you need to know for a level. And why is the equivalence point not always at ph7? in. Titration Curve How To Read.
From mungfali.com
Titration Curve Labeled Titration Curve How To Read in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? The equivalence point of a. in this section, we are going to. Titration Curve How To Read.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher ionic equilibrium titration curves Titration Curve How To Read Strong acid/strong base titration curves. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The equivalence point of a. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution.. Titration Curve How To Read.
From ceckgokq.blob.core.windows.net
Titration Curve In Biochemistry at William Speece blog Titration Curve How To Read a titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a level. The figure below shows two different examples of a. how do you explain the shape of a titration curve? The equivalence point of a. The shape of the graph produced is called a titration.. Titration Curve How To Read.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve How To Read The figure below shows two different examples of a. The equivalence point of a. And why is the equivalence point not always at ph7? in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. a titration curve is a graphical representation of the ph of. Titration Curve How To Read.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curve How To Read how do you explain the shape of a titration curve? You will also learn about equivalence points and. in this section, we are going to learn how to interpret titration curves and use them to predict what is reacting in solution. The equivalence point of a. The figure below shows two different examples of a. in this. Titration Curve How To Read.
From exojtabwh.blob.core.windows.net
Titration Diprotic Acid at Amy Long blog Titration Curve How To Read Everything you need to know for a level. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. And why is the equivalence point not always at ph7? in this section, we are. Titration Curve How To Read.